
CE marked medical device product
This collage is about the knowledge needed to get a CE marked medical device on the EU market. Emphasis is on the Quality Management System (QMS) and Summary Technical Documentation (STED).
The search engine is using the following websites.
- ec.europa.eu/growth/sectors/medical-devices
- www.iso.org
Process analysis
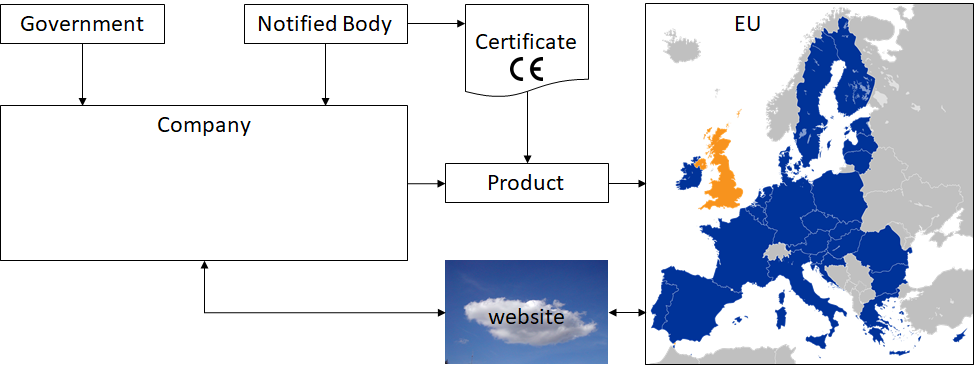
When a company manufactures a product intended for the EU market, the following have to be considered:
- the government has regulations related to medical devices
- because the product is meant for the EU market, EU regulations have to be followed
- to get a CE certificate, a Notified Body needs to be involved who will audit the company regarding it's Quality Management System and STED (Summary Technical Documentation)
- communication with (potential) customers is needed e.g. via a website, for post market surveillance, complaint handling and to make information regarding the product available (e.g. CE certificate, manuals, etc.)
Knowledge identification
Based on the process analysis about getting a CE marked product on the EU market, knowledge about STED and Quality Management System get the focus. The following websites have important information:
- Medical Devices (It is important to check this website regularly for changes on regulations.)
- Council Directive 93/42/EEC of 14 June 1993 concerning medical devices
- List of guidance MEDDEVs
- What the Notified Bodies do: Conformity Assessment
- A common framework for the marketing of products (including technical documentation)
- ISO 13485 Medical Devices
ISO 13485, Medical devices – Quality management systems – Requirements for regulatory purposes, is an internationally agreed standard that sets out the requirements for a quality management system specific to the medical devices industry.
It is designed to be used by organizations throughout the life cycle of a medical device, from initial conception to production and post-production, including final decommission and disposal. It also covers aspects such as storage, distribution, installation and servicing, and the provision of associated services.
Knowledge conversion
In the step Knowledge Identification, it is decided to put the focus on Summary TEchnical Documentation and Quality Management System for Medical Devices.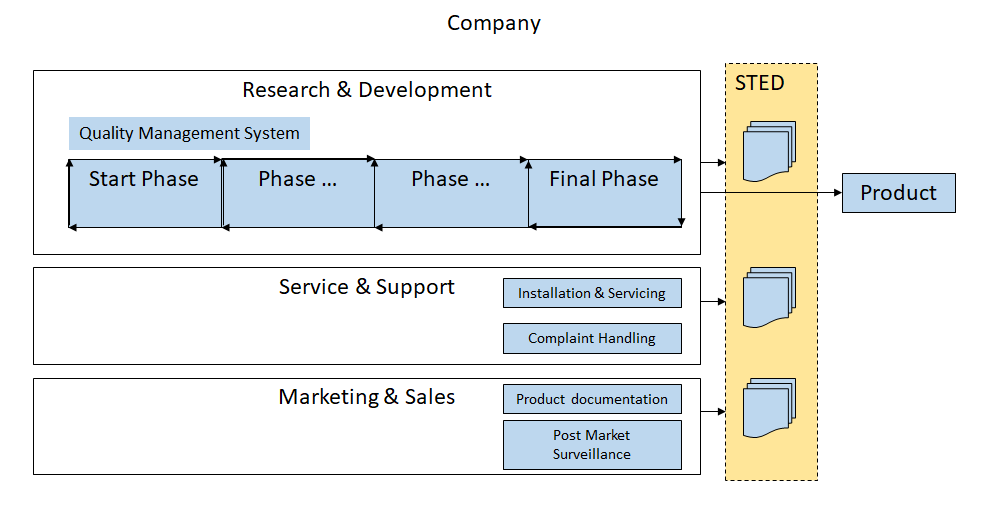
Explanation
- Research & Development is responsible for the Quality Management System and controls the STED (Summary Technical Documentation)
- The product development process is a cyclic process (each phase can be revisited depending on circumstances, e.g. change in requirements)
- Service & Support and Marketing & Sales will take care of certain phases after final phase of the product development process
- documents for the STED will be created by Research & Development, Service & Support, Marketing & Sales
- A Notified Body will do a Conformity Assessment, it will audit the company that manufactures the medical device, it's QMS and the STED See also: What the Notified Bodies do: Conformity Assessment
Externalization: from tacit to explicit knowledge conversion
Based on the information and understanding about the Conformity Assessment during the Socialization step,
the R&D Manager can adapt the product development lifecycle process where needed.
The product life cycle process will be presented and explained:
- Research & Development explain the purpose of the STED, which documents must be created and at which phase in the product development process
- Service & Support explain the purpose of Installation & Servicing and Complaint Handling
- Marketing & Sales explain the purpose of Product Documentation and Post Market Surveillance
Combination: from explicit to explicit knowledge conversion
During the product lifecycle (which is a continuous cycle) the product will be developed, changes will be made and product versions will be released.
The following knowledge will be used:
- Specific knowledge will be used depending on the product to be developed
- Standards and regulations applicable for the product
- MEDDEV
- ISO 13485
- ISO 9001
Internalization: explicit to tacit knowledge conversion
When the product has been developed, R&D will make their knowledge about the product available for Service & Support and Marketing & Sales via:
- demonstrations
- presentations
- meetings with product lead developers/engineers
- documents in STED
- preparations for Installation & Servicing the product at the customer site
- preparations for Complaint Handling
- providing any extra information or support they need in the marketing & sales of the product (e.g. at exhibitions)
- creating Product Documentation specifically for the customer (e.g. User Manual)
- Post Market Surveillance (what are the experiences of the customers with the product)